As artificial intelligence (AI) has advanced in the last decade, artificial intelligence and machine learning (AI/ML)-enabled medical devices have become a topic that is impossible to ignore in the life science industry. At NyquistAI, we maintain a weekly updated list of AI/ML-enabled devices. By 07/15/2024, a total of 942 FDA-approved devices are on the list.
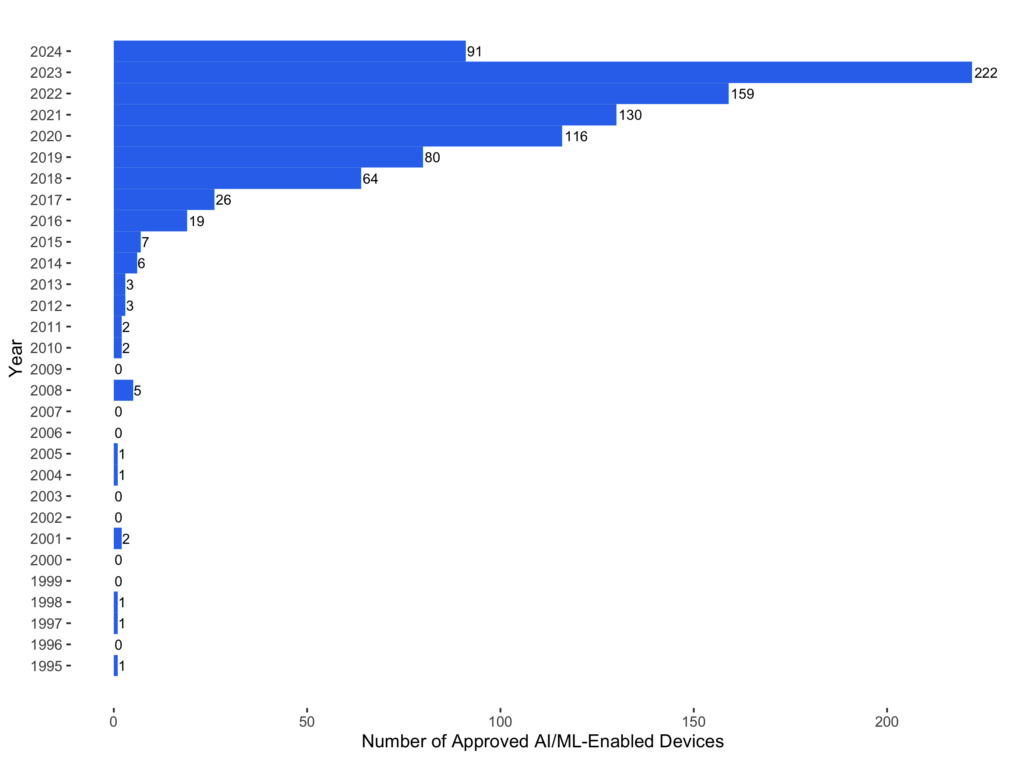
More than 50% (472 out of 942) of AI-enabled devices were approved in the last three years, and the number of approved devices is clearly increasing every year. It can be surprising that the earliest AI-enabled device was approved by the FDA in 1995. It is BD’s PAPNET Testing System (P940029, approved on 11/08/1995), which uses neural networks to detect cervical smear abnormalities.
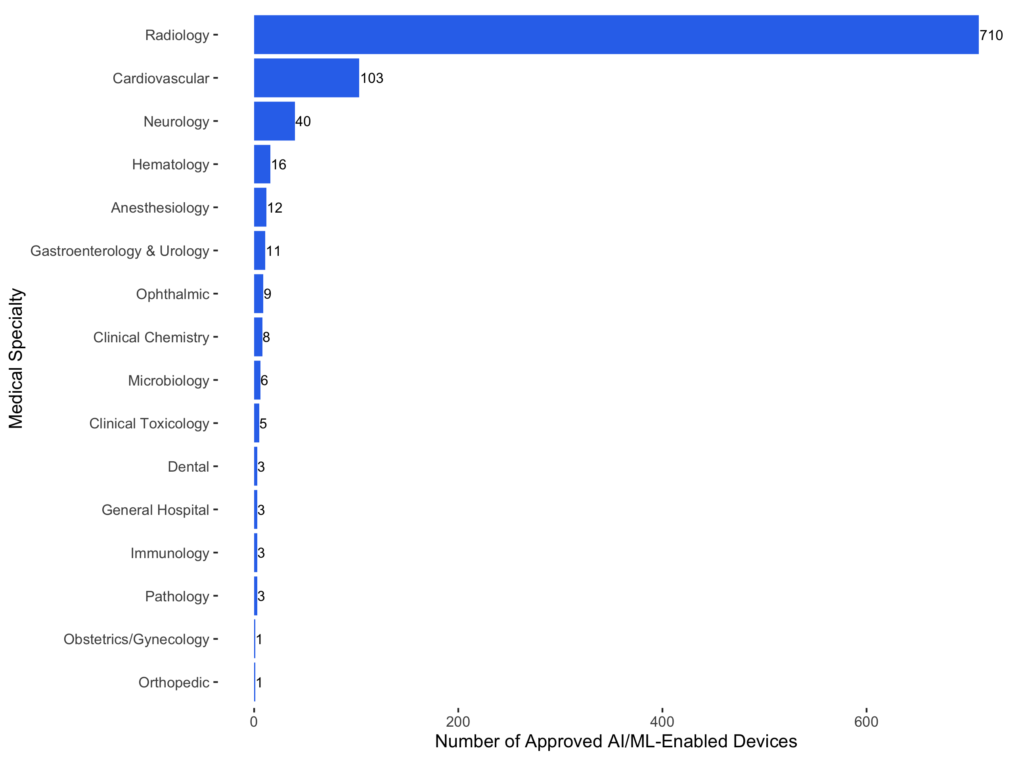
Although the first AI-enabled device was in pathology, radiology has become the dominant medical specialty in AI-enabled devices. The first radiological AI device is the M1000 IMAGECHECKER (P970058, approved on 06/26/1998) from Hologic. More than 75% (710 of 942) of AI-enabled devices belong to radiology. Radiology itself has also become increasingly like an AI specialty. In 2024, the FDA approved 208 radiological devices, and 66 (around 32%) have AI components. The ratios are similar in 2022 (134 of 411) and 2023 (175 of 450).
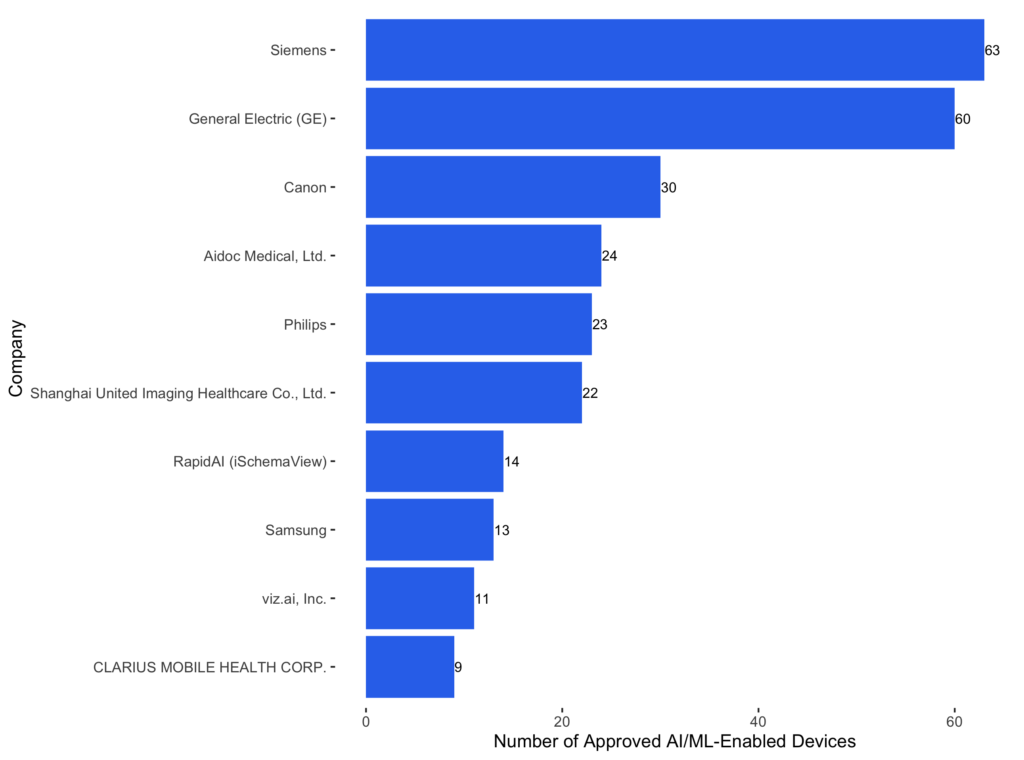
Since most AI-enabled devices belong to the radiology specialty, we expect to see major radiological manufacturers in the top 10 companies. Siemens and GE HealthCare are at the top, and both received significantly more approval than others. The top 10 companies can be categorized into two classes: traditional radiological manufacturers, like Siemens and GE, and newly founded AI startups, like Aidoc and Viz.ai. The former integrates AI features into their radiological devices, and the latter focuses on building AI solutions from scratch. Almost all approved devices from those startups have AI components.
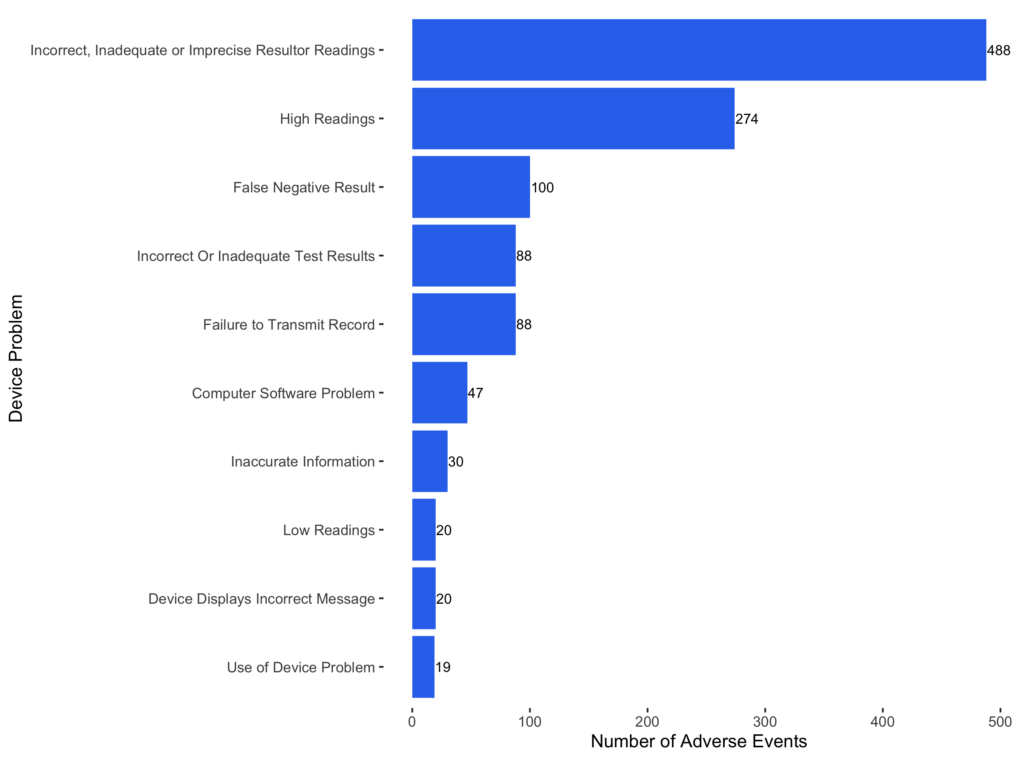
Among all 942 AI-enabled devices, 57 have been reported in 1,109 adverse event reports. The majority of the device problems are related to device reading and incorrect reporting.
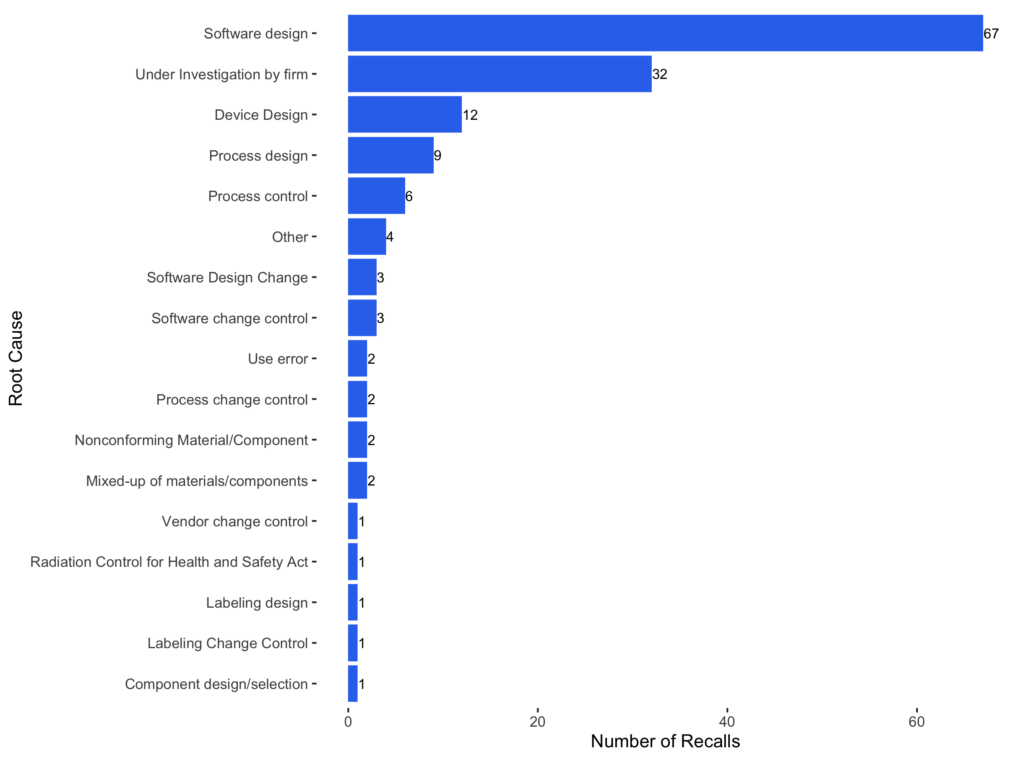
Similarly, 51 AI-enabled devices were reported in 149 recalls. The software components play a crucial role in AI-enabled devices, and software issues are the primary root cause of those recalls.